Abstract
Background: ASTX727, an oral formulation of the fixed dose combination of decitabine and cytidine deaminase inhibitor cedazuridine (35 mg/100 mg), has been recently approved for the treatment of patients (pts) with myelodysplastic syndrome. Combination of the hypomethylating agents (decitabine or azacytidine) with venetoclax (Ven), an orally bioavailable BCL-2 inhibitor, is the standard of care for treating pts with AML unfit for intensive induction chemotherapy based on age and/or comorbidity. We are investigating whether a total oral therapy regimen of ASTX727+ven is feasible and safe.
Methods: Pts aged ≥18 years with relapsed/refractory AML (R/R) or pts with AML (excluding acute promyelocytic leukemia) aged ≥ 75 or 18 -74 with comorbid conditions prohibiting intensive chemotherapy (frontline, FL) are eligible to participate. Other eligibility criteria included adequate renal and hepatic function and absence of uncontrolled infections or other uncontrolled comorbidities and an ECOG performance status (PS) of ≤2. ASTX727 is administered orally daily on days 1-5 of each treatment cycle and Ven on days 1-28 of the first cycle after a dose ramp up of 100-200-400 mg over 3 days (with tumor lysis prophylaxis precautions and with ven dose adjustments as needed based on concurrent CYP3A4 inhibitor therapy such as azole antifungals). A bone marrow exam is performed on day 21±3 days of first cycle and ven is held if blasts cleared to <5% to allow count recovery. Bone marrow exam is repeated on day 28±7 days to document response. Cycles are repeated every 4-8 weeks depending on count recovery and venetoclax is administered for 21 days in subsequent cycles. Dose adjustments of both ASTX727 and ven are permitted depending on tolerance.
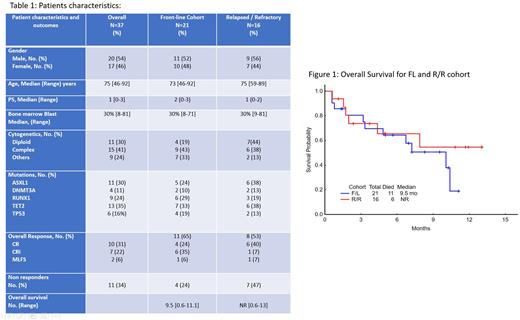
Results: As of July 11th, 2022, a total of 37 pts (21 FL, 16 R/R) have been enrolled on the study. The median age is 75 years (range, 46-92); 73 in the FL cohort and 75.5 in the R/R cohort. Nine (43%) frontline pts were older than 75 years including 8 pts (38%) ≥80 years. In the R/R cohort, 11 pts (69%) were 70-80 years. The median PS was 1 (range 0-3). The R/R cohort had a median 2 of prior treatments (range, 1-4). In the frontline cohort, 4 pts (19%) had normal cytogenetics, 9 (43%) a complex karyotype; and 7 (33%) other abnormalities including trisomy 8 and miscellaneous. In the R/R cohort, 44% had normal karyotype, 38% complex karyotype and 13% other. Mutations of note in the frontline cohort were TET2 (35%), ASXL1 (30%), RUNX1 (24%), TP53 (16%), and DNMT3A (11%).
The overall response including complete response (CR), CR with incomplete count recovery (CRi) and morphological leukemia free state (MLFS) in the frontline cohort is 65% (4 CR, 6 CRi, 1 MLFS and 4 non-responders and 2 not evaluable). 2 pts received only one day of therapy for severe adverse events unrelated to therapy (1 due to ischemic stroke, 1 due to septic shock) and were not evaluable for response. In the R/R cohort, the overall response rate is 53% (6 CR, 1 CRi, 1 MLFS with 7 non-responders). Both cohorts received a median of 3 cycles (range for both cohorts, 1-8). With a median follow-up of 7 months, the median survival for the FL cohort is 9.5 months (range, 0.6 - 11.1) and has not been reached for the R/R cohort (range, 0.6 - 13).
Grade 3 or higher adverse events related to study drugs were mainly myelosuppression-related; 4 patients (11%) had infection complications, including 1 pt with life-threatening pneumonia, and 1 pt (3%) who had liver enzyme abnormality.
Conclusion: ASTX727 and venetoclax combination is safe and feasible particularly in the advanced elderly population and demonstrates significant efficacy in pts unfit for chemotherapy both in the FL and R/R settings. Total oral therapy of ASTX727 and venetoclax combination appears to be a promising strategy for older and unfit patients with AML.
Disclosures
Garcia-Manero:Acceleron Pharma: Consultancy; BMS: Consultancy, Honoraria, Research Funding; Aprea: Honoraria; Curis: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; Gilead Sciences: Research Funding; Genentech: Honoraria, Research Funding; Astex: Consultancy, Honoraria, Research Funding. Short:Takeda Oncology: Consultancy, Research Funding; Stemline Therapeutics: Research Funding; Astellas: Research Funding; Pfizer: Consultancy; Novartis: Consultancy; Amgen: Consultancy, Honoraria; AstraZeneca: Consultancy. Alvarado:Astex Pharmaceuticals: Research Funding; Daiichi-Sankyo/Lilly: Research Funding; BerGenBio: Research Funding; Sun Pharma: Research Funding; FibroGen: Research Funding; Jazz Pharmaceuticals: Research Funding. Issa:Novartis, Kura Oncology, Nuprobe: Consultancy; Celgene, Kura Oncology, Syndax, Merck, Cullinan and Novartis: Research Funding. Yilmaz:Daiichi-Sankyo: Research Funding; Pfizer: Research Funding. Jain:Precision Biosciences: Consultancy, Honoraria, Other: Travel Support, Research Funding; Ipsen: Honoraria; MEI Pharma: Honoraria; Incyte Corporation: Research Funding; AbbVie: Consultancy, Honoraria, Other: Travel Support, Research Funding; Dialectic Therapeutics: Research Funding; Newave: Research Funding; Aprea Therapeutics: Research Funding; TG Therapeutics: Honoraria; Cellectis: Honoraria, Research Funding; Pfizer: Research Funding; Mingsight: Research Funding; TransThera Sciences: Research Funding; Genentech, Inc.: Consultancy, Honoraria, Other: Travel Support, Research Funding; CareDx: Honoraria; Servier Pharmaceuticals LLC: Research Funding; Pharmacyclics, Inc.: Consultancy, Honoraria, Other: Travel Support, Research Funding; Kite, a Gilead Company: Consultancy, Honoraria, Research Funding; Loxo Oncology: Research Funding; BMS: Consultancy, Honoraria, Other: Travel Support, Research Funding; Medisix: Research Funding; Beigene: Honoraria; Janssen Pharmaceuticals, Inc.: Consultancy, Honoraria, Other: Travel Support; Takeda: Research Funding; Adaptive Biotechnologies: Consultancy, Honoraria, Other: Travel Support, Research Funding; AstraZeneca: Consultancy, Honoraria, Other: Travel Support, Research Funding; Fate Therapeutics: Research Funding; Cellectis: Honoraria, Research Funding; Novalgen: Research Funding; ADC Therapeutics: Research Funding. Jabbour:Adaptive Biotechnologies: Other: Advisory Role, Research Funding; Genentech: Other: Advisory Role, Research Funding; AbbVie: Other: Advisory Role, Research Funding; Takeda: Other: Advisory Role, Research Funding; Spectrum: Research Funding; Amgen: Other: Advisory Role, Research Funding; Bristol Myers Squibb: Other: Advisory Role, Research Funding; Pfizer: Other: Advisory Role, Research Funding. Pemmaraju:stemline: Consultancy; abbvie: Consultancy; immunogen: Consultancy; mustangbio: Research Funding; incyte: Consultancy; novartis: Research Funding; pacylex: Consultancy, Research Funding; samus: Research Funding; daiichi sankyo: Research Funding; cellectis: Research Funding; cellularity: Research Funding. DiNardo:Novartis: Honoraria; GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees; Kura: Honoraria, Membership on an entity's Board of Directors or advisory committees; Foghorn: Honoraria, Research Funding; Gilead: Honoraria; AbbVie: Consultancy, Research Funding; GenMab: Membership on an entity's Board of Directors or advisory committees; Forma: Research Funding; Jazz: Honoraria; Cleave: Research Funding; Bluebird Bio: Honoraria; Bristol Myers Squibb: Honoraria, Research Funding; Notable Labs: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Servier: Consultancy, Honoraria, Research Funding; Astellas: Honoraria; ImmuneOnc: Honoraria, Research Funding; Astex: Research Funding; LOXO: Research Funding; Takeda: Honoraria. Kadia:Astex: Honoraria; Pfizer: Research Funding; cellenkos: Research Funding; cyclacel: Research Funding; Amgen: Research Funding; Ascentage: Research Funding; Servier: Consultancy; Glycomimetics: Research Funding; Astellas: Research Funding; AstraZeneca: Research Funding; Genfleet: Research Funding; Iterion: Research Funding; PinotBio: Consultancy; Regeneron: Research Funding; Delta-Fly: Research Funding; BMS: Consultancy, Research Funding; Novartis: Consultancy; JAZZ: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; Agios: Consultancy; Abbvie: Consultancy, Research Funding. Daver:Agios, Celgene, SOBI and STAR Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Kartos and Jazz Pharmaceuticals: Other: Data monitoring committee member; Karyopham Therapeutics and Newave Pharmaceutical: Research Funding; Astellas, AbbVie, Genentech, Daiichi-Sankyo, Novartis, Jazz, Amgen, Servier, Karyopharm, Trovagene, Trillium, Syndax, Gilead, Pfizer, Bristol Myers Squibb, Kite, Actinium, Arog, Immunogen, Arcellx, and Shattuck: Consultancy, Other: Advisory Role; Astellas, AbbVie, Genentech, Daiichi-Sankyo, Gilead, Immunogen, Pfizer, Bristol Myers Squibb, Trovagene, Servier, Novimmune, Incyte, Hanmi, Fate, Amgen, Kite, Novartis, Astex, KAHR, Shattuck, Sobi, Glycomimetics, Trillium: Research Funding. Konopleva:Sanofi: Research Funding; Rafael Pharmaceutical: Research Funding; AstraZeneca: Research Funding; Ascentage: Research Funding; Ablynx: Research Funding; Calithera: Research Funding; Cellectis: Research Funding; Eli Lilly: Consultancy, Honoraria, Research Funding; Stemline therapuetics: Consultancy, Honoraria, Research Funding; F. Hoffman La-Roche: Consultancy, Honoraria, Research Funding; Genentech: Consultancy, Honoraria, Research Funding; Abbvie: Consultancy, Honoraria, Research Funding; Forty Seven: Honoraria, Research Funding; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees. Kantarjian:KAHR Medical Ltd: Honoraria, Membership on an entity's Board of Directors or advisory committees; Ipsen Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees; NOVA Research: Honoraria; Ascentage: Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Honoraria, Research Funding; Jazz Pharmaceuticals: Research Funding; AbbVie: Honoraria, Research Funding; ImmunoGen: Research Funding; Daiichi-Sankyo: Consultancy, Research Funding; Astellas Health: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Research Funding; Pfizer: Honoraria, Research Funding; Takeda: Honoraria. Ravandi:Astex/Taiho: Membership on an entity's Board of Directors or advisory committees, Research Funding; AstraZeneca: Consultancy; Prelude: Research Funding; Syos: Consultancy, Honoraria, Research Funding; Abbvie: Consultancy, Honoraria, Research Funding; Xencor: Research Funding; Amgen: Honoraria, Research Funding; Biomea Fusion, Inc.: Research Funding; Novartis: Consultancy; Astellas: Consultancy, Honoraria, Research Funding; BMS/Celgene: Consultancy, Honoraria, Research Funding; Amgen: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal